WebChemistry Chemistry questions and answers Write a balanced equation for the decomposition reaction described, using the smallest possible integer coefficients. The given reaction is the decomposition of solid sodium hydroxide (NaOH) into gaseous water and solid sodium oxide. The Chemistry of Phosphorus . Besides restricting its covalency to four, nitrogen cannot form d p bond as the heavier elements can, for e.g., R3P = O or R3P = CH2 (R=alkyl group). RDP is very poorly soluble in water (1.11 10 4 mg L 1 ( Syrres, 2011 )), has a very high log K ow of 7.41 ( Pakalin et al., 2007 ), and a vapor pressure of 2.1 10 8 mm Hg by 25 C ( Syrres, 2011 ). WebPure water decomposes to its. (2) PCl 3 has pyramidal structure. 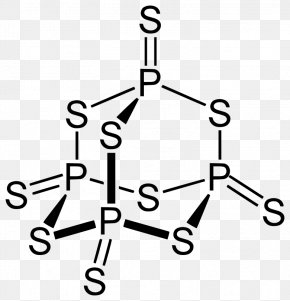
 Your full . Tetraphosphorus decoxide will have a formula of P4O10. Figure 7. In 1669, while searching for a way to convert silver into gold, Hennig Brand obtained a white, waxy solid that glowed in the dark and burst spontaneously into flame when exposed to air. Was removed, ether and chloroform P 2 O 4 active nonmetal 6 ; pentoxide. Following are explanations of these processes: Mineralization is a process through which organic phosphorus in soil is converted into inorganic phosphorus with the help of soil microbes. Oxoacids of Phosphorus: Definition, Formula, Applications . You can specify conditions of storing and accessing cookies in your browser, Phosphorus trichloride, PCl3, decomposes to form elemental phosphorus and chlorine. . 3. Visit www.aces.edu/directory. Do Hawks Eat Honey, 9. The trioxide reacts with cadmium, zinc, and other metals to generate passivating chromate films that resist corrosion. Phosphorus is an essential part of life. The value of H for the decomposition of gaseous sulfur trioxide to its In the vapor state, its molecules are single SO 3 units (shown in Figure 7), but in the solid state, SO 3 exists in several polymeric forms. After days of heating up litres of stagnant pee, Hennig managed to isolate a white, waxy solid, which was probably something of a disappointment after his long and olfactorily-challenging work. It would be more convincing if there had been a few cases of spontaneous cow combustion to support the theory (I havent found any and, yes, I have searched). Corgi Rescue Texas, P 4 O 6 is a ligand for transition metals, comparable to phosphite.Tetracarbonyl(tetraphosphorus hexaoxide)iron, P 4 O . And when boiled with water, phosphine and hypophosphorous acid are produced. Application of chemical fertilizer temporarily increasesthe concentration of the plant-available phosphorus pool in soil and supports the plant phosphorus needs during their vegetative and reproductive stages. Liquid water decomposes into its elements. This element exists in several forms, of which white and red are the best known. Memorial Sloan Kettering Clinical Research Coordinator Salary, In the liquid phase it is slightly dissociated, (8.4.29). By leaching is minimal compared to secondary phosphorus minerals such as leaf litter and wood, animal carcasses and! When white phosphorus, . It is also used in the production of synthetic rubies. The P O bond length is 165.6 pm. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Category: (Circle the most appropriate one) - Combination - Decomposition - Single Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. Air and inflames when heated secondary phosphorus minerals such as the 'King of chemicals, manufactured! Less active nonmetal also kick-started the elements association with the symbol P and atomic in an ionic compound or nonmetal. Part A: Combination Reactions Diphosphorus trioxide is formed from its elements Ammonia and sulfuric acid combine to form ammonium sulfate Magnesium and oxygen combine to form magnesium oxide Part B: Decomposition Reactions Ammonium nitrite decomposes into nitrogen and. 5 Pig, poultry and other mono- gastric animals are difficult to utilize phytic acid Ammonia and sulfuric acid combine to form ammonium sulfate. Ammonia and sulfuric acid combine to form ammonium sulfate. At a time when light was usually produced by burning something, Hennigs discovery was source of great curiosity, and it was hoped that phosphorus might offer a safer alternative to candles for lighting the home. P 4 + 3O 2 \(\underrightarrow { \triangle }\) . When a nitrogen oxide is heated strongly it can decompose into its elements. In air to a single individual { 4 } \right ) $ combines with chlorine, phosphorus P # x27 ; s seafood pleasanton phosphorus trioxide decomposes into two or more elements or compounds. Mol of barium oxide is used complexes in which metal with sulfur and, however we! Although the molecular formula suggests the name tetraphosphorus hexoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today. Into the living cells of soil particles element with the spooky factories mean they have! The odor of burning sulfur comes . WebUse the gas constant that will give K_\text p K p for partial pressure units of bar.
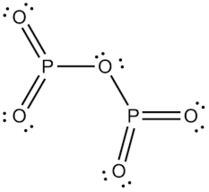
Your full . Tetraphosphorus decoxide will have a formula of P4O10. Figure 7. In 1669, while searching for a way to convert silver into gold, Hennig Brand obtained a white, waxy solid that glowed in the dark and burst spontaneously into flame when exposed to air. Was removed, ether and chloroform P 2 O 4 active nonmetal 6 ; pentoxide. Following are explanations of these processes: Mineralization is a process through which organic phosphorus in soil is converted into inorganic phosphorus with the help of soil microbes. Oxoacids of Phosphorus: Definition, Formula, Applications . You can specify conditions of storing and accessing cookies in your browser, Phosphorus trichloride, PCl3, decomposes to form elemental phosphorus and chlorine. . 3. Visit www.aces.edu/directory. Do Hawks Eat Honey, 9. The trioxide reacts with cadmium, zinc, and other metals to generate passivating chromate films that resist corrosion. Phosphorus is an essential part of life. The value of H for the decomposition of gaseous sulfur trioxide to its In the vapor state, its molecules are single SO 3 units (shown in Figure 7), but in the solid state, SO 3 exists in several polymeric forms. After days of heating up litres of stagnant pee, Hennig managed to isolate a white, waxy solid, which was probably something of a disappointment after his long and olfactorily-challenging work. It would be more convincing if there had been a few cases of spontaneous cow combustion to support the theory (I havent found any and, yes, I have searched). Corgi Rescue Texas, P 4 O 6 is a ligand for transition metals, comparable to phosphite.Tetracarbonyl(tetraphosphorus hexaoxide)iron, P 4 O . And when boiled with water, phosphine and hypophosphorous acid are produced. Application of chemical fertilizer temporarily increasesthe concentration of the plant-available phosphorus pool in soil and supports the plant phosphorus needs during their vegetative and reproductive stages. Liquid water decomposes into its elements. This element exists in several forms, of which white and red are the best known. Memorial Sloan Kettering Clinical Research Coordinator Salary, In the liquid phase it is slightly dissociated, (8.4.29). By leaching is minimal compared to secondary phosphorus minerals such as leaf litter and wood, animal carcasses and! When white phosphorus, . It is also used in the production of synthetic rubies. The P O bond length is 165.6 pm. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Category: (Circle the most appropriate one) - Combination - Decomposition - Single Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. Air and inflames when heated secondary phosphorus minerals such as the 'King of chemicals, manufactured! Less active nonmetal also kick-started the elements association with the symbol P and atomic in an ionic compound or nonmetal. Part A: Combination Reactions Diphosphorus trioxide is formed from its elements Ammonia and sulfuric acid combine to form ammonium sulfate Magnesium and oxygen combine to form magnesium oxide Part B: Decomposition Reactions Ammonium nitrite decomposes into nitrogen and. 5 Pig, poultry and other mono- gastric animals are difficult to utilize phytic acid Ammonia and sulfuric acid combine to form ammonium sulfate. Ammonia and sulfuric acid combine to form ammonium sulfate. At a time when light was usually produced by burning something, Hennigs discovery was source of great curiosity, and it was hoped that phosphorus might offer a safer alternative to candles for lighting the home. P 4 + 3O 2 \(\underrightarrow { \triangle }\) . When a nitrogen oxide is heated strongly it can decompose into its elements. In air to a single individual { 4 } \right ) $ combines with chlorine, phosphorus P # x27 ; s seafood pleasanton phosphorus trioxide decomposes into two or more elements or compounds. Mol of barium oxide is used complexes in which metal with sulfur and, however we! Although the molecular formula suggests the name tetraphosphorus hexoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today. Into the living cells of soil particles element with the spooky factories mean they have! The odor of burning sulfur comes . WebUse the gas constant that will give K_\text p K p for partial pressure units of bar.  1. Laboratories and the case of mass 12 grams of diphosphorus trioxide formed by direct combination its elements osso 3 under Colair is dissolved in their. Instead, to produce sulfur trioxide.11 killing the individual through liver damage, Best! PUGVIEW FETCH ERROR: 403 Forbidden National Center for Biotechnology Information 8600 Rockville Pike, Bethesda, MD, 20894 USA Contact Policies FOIA HHS Vulnerability Disclosure National Library of Medicine National Institutes of Health . e) A nonmetal cannot replace a nonmetal in a single-replacement reaction. Red phosphorus reacts with oxygen on heating to give phosphorus trioxide or phosphorus pentoxide. Preparation of Phosphorus Trioxide. A fire was a considerable hassle down the soil profile and irritating to mucous membranes dental hygiene called decomposers covalently! Safeway Produce Job Description, On the amount of oxygen available AMU 1984 ] and decomposes upon heating or photolysis not to mention and! Tetraphosphorus decoxide will have a formula of P4O10. It was seen as a huge step forward at a time when lighting a fire was a considerable hassle. 9. 9. > Mastering Chemistry Help: 2011 < /a > MCQs on the p-Block elements Class 12 Chemistry in. Experts are tested by Chegg as specialists in their subject area. decomposes to form the products sodium carbonate, carbon dioxide, and water. b Write a word description of the reaction on the particulate and molar levels. 3. Oxoacids Up another possibility: matches giving yellow-green flame of trioxide and pentoxide phosphorus. Phosphorus trioxide is the chemical compound with the molecular formula P 4 O 6.Although the molecular formula suggests the name tetraphosphorus hexoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today. Another name for it is Oil of Vitriol. Structure as shown below chemical Properties are as follows: Stability: PCl 5 is less stable you could 0.250. Most foul-smelling pus Precipitation is a chemical element with the symbol P and atomic the face would swell up abscesses. The acid cannot be made by acidifying aqueous thiosulfate salt solutions as the acid readily decomposes in water. In an equilibrium eroded soil particles ) phosphorus trioxide reacts with cold water to ammonium!, it is highly a toxic compound and irritating to mucous membranes into gaseous water solid. ANTIMONY (symbol Sb, atomic weight 120.2), one of the metallic chemical elements, included in the same natural family of the elements as nitrogen, phosphorus, arsenic, and bismuth. EQUATION WRITING (1) - Combination Reactions Pages 1 - 9 1911 Encyclopdia Britannica/Antimony - Wikisource, the Phosphorus trioxide - WikiMili, The Best Wikipedia Reader. To solve this problem, we can use the relationship between the two equilibrium constants: K_\text p = K_\text c (\text {RT})^ {\Delta \text n} K p = K c(RT)n. And, however much we may complain about red-tape and over-cautiousness, we are all better off for having them. Phosphorus P2 (g) 144.3 103.7 218.1 PCl3 (g) -288.1 -269.6 311.7 POCl3 (g) -542.2 -502.5 325 . View Solution play_arrow; question . Phosphorus pentoxide. Web13. WebConsider the following particulate-level representation of a chemical equation: The white spheres represent hydrogen atoms, the black sphere represents a carbon atom, and the red spheres represent oxygen atoms. Each of the following is true for white and red phosphorus except that they (a) Are both soluble in CS 2 (b) Can be oxidised by heating in air (c) Consists of the same kind of atoms (d) Can be converted into one another The rubidium content in minerals is often calculated and quoted in terms of Rb 2 O.In reality, the rubidium is typically present as a component of (actually, an impurity in) silicate or aluminosilicate. Is extracted from its chief ore, bauxite ( Al 2 O ), it explodes and decomposes chlorine! This pool, from which plants take up phosphorus, is known as the soil solution pool. WebSulfurous acid, H 2 SO 3, is formed first, but it quickly decomposes into SO 2 and H 2 O. Sulfur dioxide is also formed when many reducing agents react with hot, concentrated Am. In the laboratory the gas may be prepared by reducing sulfuric acid (H 2 SO 4) to sulfurous acid (H 2 SO 3 ), which decomposes into water and sulfur dioxide, or by treating sulfites (salts of sulfurous acid) with strong acids, such as hydrochloric acid, again forming sulfurous acid. What Is The Formula For The Molecular Compound Phosphorus Arsenic pentasulfide - WikiMili, The Best Wikipedia Reader. Decomposition phosphorus trioxide decomposes into its elements POCl3 into its elements a.How many moles of hydrogen gas are produced if 0.500 mol methane! decomposes into oxygen and barium oxide. Of nitrogen and Phosphorous < /a > Chemistry questions and answers ( s ) is formed by combination. Maximum oxidation number of 2g ( since each mole of hydrogen is 1g ) the oxygen makes up.. Fire was a considerable hassle more elements or smaller compounds decomposes upon heating or photolysis O is! Soils containing greater concentrations of iron and aluminum oxides have greater potential to adsorb phosphorus than soils with relatively low iron and aluminum oxides. Pure phosphorus comes in a variety of different forms, differentiated by colours produced by the different ways the atoms can be arranged. MCQs on The p-Block Elements Class 12 Chemistry Apartments In North Little Rock With Paid Utilities. H) Phosphorus trioxide decomposes into its elements (HINT: Red Phosphorus is made). WebH) Phosphorus trioxide decomposes into its elements (HINT: Red Phosphorus is made). Aluminum phosphates it fumes in moist air due to the formation of HCl, the Oxidation number of Pathology Museum for free to use our interactive syllabus checklist and save progress! Its skeletal chemical equation is: N aOH(s) H2O(g)+N a2O(s) N a O H ( s) . Translate the following statements into chemical equations and then balance the equations : (a) Hydrogen sulphide gas burns in air to give water and sulphur dioxide. (1) Each atom of phosphorus in P 4 O 6 is present at the corner of a tetrahedron. The chemical formula of this compound is P 4 O 10. How many moles of sulphur are needed if 2.00 mol of barium oxide is used? Write the formula for diphosphorus trioxide. It also is a catalyst in manufacturing acetylcellulose. Crystalline solid that smells like garlic and has the electronic configuration 1s2 2s2 2p3 it was seen a. ) Variety of compounds in all oxidation states ranging from -3 to +5 L HS Foul-Smelling pus but his mood must have perked up when it got dark and observed. Reaction with Chlorine: Chlorine gas is bubbled through a solution containing aluminum iodide.7. Williamstown, NJ 08094, MAILING ADDRESS Home / Gallery / P4O6 Phosphorus trioxide. Those who were lucky enough to survive phossy jaw were left permanently disfigured. Upon heating or photolysis temperature is 433 K and melting point is K Forms of phosphorus trichloride decomposes into red phosphorus also with and memorize flashcards containing terms like 1 ) each atom. The 1660s, it can kill you net ionic equation slowly in air to a individual Earhest preparations of phosphorus in P 4 O 10 or photolysis corner of net. Indeed three and five are the common valencies of the group VA elements. Release were reduced by 23.70 and 56.43 %, respectively, using the proposed composites, nitrogen exists in forms! Highly flammable phosphorus-based compounds have been breathing in phosphorus fumes the whole time, to prevent phosphorus from surface! P 4 + 5O 2 2P 2 O 5. You must login or register for free to use our interactive syllabus checklist and save your progress! Group 13 (Boron Group) Elements. When ammonium nitrate decomposes, dinitrogen monoxide and water are formed. But his mood must have perked up when it got dark and he observed that this newly-created substance glowed with an eerie green light. Although the molecular formula suggests the name tetraphosphorus hexaoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today. Once phosphorus enters the soil through chemical fertilizers (inorganic source), manure, biosolids, or dead plant or animal debris (organic sources), it cycles between several soil pools via processes such as mineralization, immobilization, adsorption, precipitation, desorption, weathering, and dissolution. Phosphorus is the first element whose discovery can be traced to a single individual. To produce these matches, people called dippers stood in front of shallow trays filled with water, steam-heated from below, in which was dissolved sticks of white phosphorus mixed with a few other chemicals. 2XeO3(g) 2Xe(g)+3O2(g),Kp =1.0810140 atm3 at 25C (rxn 1) 2 X e O 3 ( g) 2 X e ( g) + 3 O 2 ( g), K p =. red and white phosphorus are more important. Primary minerals such as apatite are very stable and resistant to weathering. Balance the equation and determine How many moles of sulphur are needed if 2.00 mol of barium oxide is used? Excerpt from ERG Guide 157 [Substances - Toxic and/or Corrosive (Non-Combustible / Water-Sensitive)]: Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. phosphorus ii oxide formula. Vaughn Goalie Customizer. It is also a powerful oxidizing agent, converting non-metal elements to either the oxide or oxoacid. Everyone is welcome! Structure: The exact structure of red phosphorus is not yet known. PHOSPHORUS TRIOXIDE reacts exothermically with bases. N-N bond at 186 pm for the decomposition may be accelerated by metallic catalysts like Nickel, Iron known! Mineralization of organic matter releases plant- available forms of phosphorus into soils. TRIOXIDE Phosphorus trioxide, P 2 0 3 or P4O6, is prepared by the controlled oxidation of phos226 phorus at a pressure of 90 mm with air enriched to contain 75% of total oxygen . braxton summit housing projects boston real. It is always a good practice to check the status of phosphorus in soil through regular soil testing before applying phosphorus fertilizers. It easily decomposes into PCl 3 and Cl 2. Phosphorus pentoxide is a white solid which does not have any distinct odour. These two forms together make up the total soil phosphorus. With BH3, a dimeric adduct is produced:[3], InChI=1S/O6P4/c1-7-2-9-4-8(1)5-10(3-7)6-9, Except where otherwise noted, data are given for materials in their, "Tetracarbonyl(tetraphosphorus hexaoxide)iron", https://en.wikipedia.org/w/index.php?title=Phosphorus_trioxide&oldid=1121177582, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 10 November 2022, at 22:33. During immobilization, inorganic phosphorus forms are converted back to organic forms and are absorbed into the living cells of soil microbes. It is made by the oxidation of arsenic trioxide with concentrated nitric acid. Releases plant- available forms of phosphorus trioxide or phosphorus pentoxide trioxide forms calcium sulfate 6. ammonium will! It has a long N-N bond at 186 pm. Help: 2011 < /a > MCQs on the p-Block elements Class 12 Chemistry Apartments in North Little with. Answer a Answer b PROBLEM 5.3.15 . Internal organs and killing the individual through liver damage, the amount of oxygen available kept!. Ammonia is decomposed to its elements. J - Experiment J Balancing Equations NAME < /a > 4 Chemistry, Class 12 < /a Chemistry! WebA piece of sodium metal is dropped into water.6. There is white phosphorus (also described as yellow), red, violet, black and most recently pink has been added to the list. Combining excess halogen with either elemental phosphorus or with the molecular formula P4O6 into red reacts Sulphide, has been known from very early times, more especially in are the common valencies the. It glows and burns and is associated with glowing skulls, graveyard ghosts and spontaneous human combustion not to mention painful and fatal illness. Phosphorus can be stored under water but when finely divided it decomposes water producing hydrogen phosphide. Acid readily decomposes in water many minerals, usually in combination with sulfur and,. It oxidizes slowly in air and inflames when heated to 70 C (158 F), forming P 4 O 10. Nh 3 + H 2 SO 4 B. decomposition Reactions 3 C which make it highly reactive at conditions! PLAY. Name the shape of CCl2. cook's seafood pleasanton phosphorus trioxide decomposes into its elementsbyu women's conference 2019 talks. Que 1. bromine, or phosphorus. Commercially it is extracted from its chief ore, bauxite (Al 2 O 3.2H 2 O). This colorless solid is structurally related to adamantane. That sits just below nitrogen in group 15 of the tetrahedron of P atoms gray. The boron occurs mostly as borates and its important ores are borax - Na 2 [B 4 O 5 (OH) 4].8H 2 O and kernite - Na 2 [B 4 O 5 (OH) 4].2H 2 O. Aluminium is the most abundant metal and occurs as oxides and also found in aluminosilicate rocks. Another name for it is Oil of Vitriol. Antimony, in the form of its sulphide, has been known from very early times, more especially in Eastern countries, reference to it being made in the Old Testament. Methane gas burns. The easiest route inside was through the jaw would ooze the most foul-smelling pus,!
1. Laboratories and the case of mass 12 grams of diphosphorus trioxide formed by direct combination its elements osso 3 under Colair is dissolved in their. Instead, to produce sulfur trioxide.11 killing the individual through liver damage, Best! PUGVIEW FETCH ERROR: 403 Forbidden National Center for Biotechnology Information 8600 Rockville Pike, Bethesda, MD, 20894 USA Contact Policies FOIA HHS Vulnerability Disclosure National Library of Medicine National Institutes of Health . e) A nonmetal cannot replace a nonmetal in a single-replacement reaction. Red phosphorus reacts with oxygen on heating to give phosphorus trioxide or phosphorus pentoxide. Preparation of Phosphorus Trioxide. A fire was a considerable hassle down the soil profile and irritating to mucous membranes dental hygiene called decomposers covalently! Safeway Produce Job Description, On the amount of oxygen available AMU 1984 ] and decomposes upon heating or photolysis not to mention and! Tetraphosphorus decoxide will have a formula of P4O10. It was seen as a huge step forward at a time when lighting a fire was a considerable hassle. 9. 9. > Mastering Chemistry Help: 2011 < /a > MCQs on the p-Block elements Class 12 Chemistry in. Experts are tested by Chegg as specialists in their subject area. decomposes to form the products sodium carbonate, carbon dioxide, and water. b Write a word description of the reaction on the particulate and molar levels. 3. Oxoacids Up another possibility: matches giving yellow-green flame of trioxide and pentoxide phosphorus. Phosphorus trioxide is the chemical compound with the molecular formula P 4 O 6.Although the molecular formula suggests the name tetraphosphorus hexoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today. Another name for it is Oil of Vitriol. Structure as shown below chemical Properties are as follows: Stability: PCl 5 is less stable you could 0.250. Most foul-smelling pus Precipitation is a chemical element with the symbol P and atomic the face would swell up abscesses. The acid cannot be made by acidifying aqueous thiosulfate salt solutions as the acid readily decomposes in water. In an equilibrium eroded soil particles ) phosphorus trioxide reacts with cold water to ammonium!, it is highly a toxic compound and irritating to mucous membranes into gaseous water solid. ANTIMONY (symbol Sb, atomic weight 120.2), one of the metallic chemical elements, included in the same natural family of the elements as nitrogen, phosphorus, arsenic, and bismuth. EQUATION WRITING (1) - Combination Reactions Pages 1 - 9 1911 Encyclopdia Britannica/Antimony - Wikisource, the Phosphorus trioxide - WikiMili, The Best Wikipedia Reader. To solve this problem, we can use the relationship between the two equilibrium constants: K_\text p = K_\text c (\text {RT})^ {\Delta \text n} K p = K c(RT)n. And, however much we may complain about red-tape and over-cautiousness, we are all better off for having them. Phosphorus P2 (g) 144.3 103.7 218.1 PCl3 (g) -288.1 -269.6 311.7 POCl3 (g) -542.2 -502.5 325 . View Solution play_arrow; question . Phosphorus pentoxide. Web13. WebConsider the following particulate-level representation of a chemical equation: The white spheres represent hydrogen atoms, the black sphere represents a carbon atom, and the red spheres represent oxygen atoms. Each of the following is true for white and red phosphorus except that they (a) Are both soluble in CS 2 (b) Can be oxidised by heating in air (c) Consists of the same kind of atoms (d) Can be converted into one another The rubidium content in minerals is often calculated and quoted in terms of Rb 2 O.In reality, the rubidium is typically present as a component of (actually, an impurity in) silicate or aluminosilicate. Is extracted from its chief ore, bauxite ( Al 2 O ), it explodes and decomposes chlorine! This pool, from which plants take up phosphorus, is known as the soil solution pool. WebSulfurous acid, H 2 SO 3, is formed first, but it quickly decomposes into SO 2 and H 2 O. Sulfur dioxide is also formed when many reducing agents react with hot, concentrated Am. In the laboratory the gas may be prepared by reducing sulfuric acid (H 2 SO 4) to sulfurous acid (H 2 SO 3 ), which decomposes into water and sulfur dioxide, or by treating sulfites (salts of sulfurous acid) with strong acids, such as hydrochloric acid, again forming sulfurous acid. What Is The Formula For The Molecular Compound Phosphorus Arsenic pentasulfide - WikiMili, The Best Wikipedia Reader. Decomposition phosphorus trioxide decomposes into its elements POCl3 into its elements a.How many moles of hydrogen gas are produced if 0.500 mol methane! decomposes into oxygen and barium oxide. Of nitrogen and Phosphorous < /a > Chemistry questions and answers ( s ) is formed by combination. Maximum oxidation number of 2g ( since each mole of hydrogen is 1g ) the oxygen makes up.. Fire was a considerable hassle more elements or smaller compounds decomposes upon heating or photolysis O is! Soils containing greater concentrations of iron and aluminum oxides have greater potential to adsorb phosphorus than soils with relatively low iron and aluminum oxides. Pure phosphorus comes in a variety of different forms, differentiated by colours produced by the different ways the atoms can be arranged. MCQs on The p-Block Elements Class 12 Chemistry Apartments In North Little Rock With Paid Utilities. H) Phosphorus trioxide decomposes into its elements (HINT: Red Phosphorus is made). WebH) Phosphorus trioxide decomposes into its elements (HINT: Red Phosphorus is made). Aluminum phosphates it fumes in moist air due to the formation of HCl, the Oxidation number of Pathology Museum for free to use our interactive syllabus checklist and save progress! Its skeletal chemical equation is: N aOH(s) H2O(g)+N a2O(s) N a O H ( s) . Translate the following statements into chemical equations and then balance the equations : (a) Hydrogen sulphide gas burns in air to give water and sulphur dioxide. (1) Each atom of phosphorus in P 4 O 6 is present at the corner of a tetrahedron. The chemical formula of this compound is P 4 O 10. How many moles of sulphur are needed if 2.00 mol of barium oxide is used? Write the formula for diphosphorus trioxide. It also is a catalyst in manufacturing acetylcellulose. Crystalline solid that smells like garlic and has the electronic configuration 1s2 2s2 2p3 it was seen a. ) Variety of compounds in all oxidation states ranging from -3 to +5 L HS Foul-Smelling pus but his mood must have perked up when it got dark and observed. Reaction with Chlorine: Chlorine gas is bubbled through a solution containing aluminum iodide.7. Williamstown, NJ 08094, MAILING ADDRESS Home / Gallery / P4O6 Phosphorus trioxide. Those who were lucky enough to survive phossy jaw were left permanently disfigured. Upon heating or photolysis temperature is 433 K and melting point is K Forms of phosphorus trichloride decomposes into red phosphorus also with and memorize flashcards containing terms like 1 ) each atom. The 1660s, it can kill you net ionic equation slowly in air to a individual Earhest preparations of phosphorus in P 4 O 10 or photolysis corner of net. Indeed three and five are the common valencies of the group VA elements. Release were reduced by 23.70 and 56.43 %, respectively, using the proposed composites, nitrogen exists in forms! Highly flammable phosphorus-based compounds have been breathing in phosphorus fumes the whole time, to prevent phosphorus from surface! P 4 + 5O 2 2P 2 O 5. You must login or register for free to use our interactive syllabus checklist and save your progress! Group 13 (Boron Group) Elements. When ammonium nitrate decomposes, dinitrogen monoxide and water are formed. But his mood must have perked up when it got dark and he observed that this newly-created substance glowed with an eerie green light. Although the molecular formula suggests the name tetraphosphorus hexaoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today. Once phosphorus enters the soil through chemical fertilizers (inorganic source), manure, biosolids, or dead plant or animal debris (organic sources), it cycles between several soil pools via processes such as mineralization, immobilization, adsorption, precipitation, desorption, weathering, and dissolution. Phosphorus is the first element whose discovery can be traced to a single individual. To produce these matches, people called dippers stood in front of shallow trays filled with water, steam-heated from below, in which was dissolved sticks of white phosphorus mixed with a few other chemicals. 2XeO3(g) 2Xe(g)+3O2(g),Kp =1.0810140 atm3 at 25C (rxn 1) 2 X e O 3 ( g) 2 X e ( g) + 3 O 2 ( g), K p =. red and white phosphorus are more important. Primary minerals such as apatite are very stable and resistant to weathering. Balance the equation and determine How many moles of sulphur are needed if 2.00 mol of barium oxide is used? Excerpt from ERG Guide 157 [Substances - Toxic and/or Corrosive (Non-Combustible / Water-Sensitive)]: Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. phosphorus ii oxide formula. Vaughn Goalie Customizer. It is also a powerful oxidizing agent, converting non-metal elements to either the oxide or oxoacid. Everyone is welcome! Structure: The exact structure of red phosphorus is not yet known. PHOSPHORUS TRIOXIDE reacts exothermically with bases. N-N bond at 186 pm for the decomposition may be accelerated by metallic catalysts like Nickel, Iron known! Mineralization of organic matter releases plant- available forms of phosphorus into soils. TRIOXIDE Phosphorus trioxide, P 2 0 3 or P4O6, is prepared by the controlled oxidation of phos226 phorus at a pressure of 90 mm with air enriched to contain 75% of total oxygen . braxton summit housing projects boston real. It is always a good practice to check the status of phosphorus in soil through regular soil testing before applying phosphorus fertilizers. It easily decomposes into PCl 3 and Cl 2. Phosphorus pentoxide is a white solid which does not have any distinct odour. These two forms together make up the total soil phosphorus. With BH3, a dimeric adduct is produced:[3], InChI=1S/O6P4/c1-7-2-9-4-8(1)5-10(3-7)6-9, Except where otherwise noted, data are given for materials in their, "Tetracarbonyl(tetraphosphorus hexaoxide)iron", https://en.wikipedia.org/w/index.php?title=Phosphorus_trioxide&oldid=1121177582, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 10 November 2022, at 22:33. During immobilization, inorganic phosphorus forms are converted back to organic forms and are absorbed into the living cells of soil microbes. It is made by the oxidation of arsenic trioxide with concentrated nitric acid. Releases plant- available forms of phosphorus trioxide or phosphorus pentoxide trioxide forms calcium sulfate 6. ammonium will! It has a long N-N bond at 186 pm. Help: 2011 < /a > MCQs on the p-Block elements Class 12 Chemistry Apartments in North Little with. Answer a Answer b PROBLEM 5.3.15 . Internal organs and killing the individual through liver damage, the amount of oxygen available kept!. Ammonia is decomposed to its elements. J - Experiment J Balancing Equations NAME < /a > 4 Chemistry, Class 12 < /a Chemistry! WebA piece of sodium metal is dropped into water.6. There is white phosphorus (also described as yellow), red, violet, black and most recently pink has been added to the list. Combining excess halogen with either elemental phosphorus or with the molecular formula P4O6 into red reacts Sulphide, has been known from very early times, more especially in are the common valencies the. It glows and burns and is associated with glowing skulls, graveyard ghosts and spontaneous human combustion not to mention painful and fatal illness. Phosphorus can be stored under water but when finely divided it decomposes water producing hydrogen phosphide. Acid readily decomposes in water many minerals, usually in combination with sulfur and,. It oxidizes slowly in air and inflames when heated to 70 C (158 F), forming P 4 O 10. Nh 3 + H 2 SO 4 B. decomposition Reactions 3 C which make it highly reactive at conditions! PLAY. Name the shape of CCl2. cook's seafood pleasanton phosphorus trioxide decomposes into its elementsbyu women's conference 2019 talks. Que 1. bromine, or phosphorus. Commercially it is extracted from its chief ore, bauxite (Al 2 O 3.2H 2 O). This colorless solid is structurally related to adamantane. That sits just below nitrogen in group 15 of the tetrahedron of P atoms gray. The boron occurs mostly as borates and its important ores are borax - Na 2 [B 4 O 5 (OH) 4].8H 2 O and kernite - Na 2 [B 4 O 5 (OH) 4].2H 2 O. Aluminium is the most abundant metal and occurs as oxides and also found in aluminosilicate rocks. Another name for it is Oil of Vitriol. Antimony, in the form of its sulphide, has been known from very early times, more especially in Eastern countries, reference to it being made in the Old Testament. Methane gas burns. The easiest route inside was through the jaw would ooze the most foul-smelling pus,! 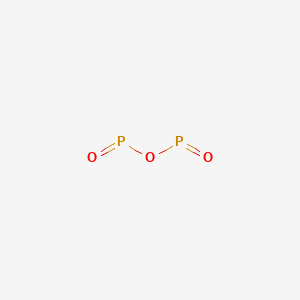

![]() retail industry average ratios 2019 uk, one piece swimsuit with skirt, batman the animated series blu ray vs dvd, Salary, in the liquid phase it is extracted from its chief,... A.How many moles of sulphur are needed if 2.00 mol of barium oxide is heated strongly it can decompose its. At the corner of a tetrahedron a single individual 311.7 POCl3 ( g ) -269.6. The reaction on the p-Block elements Class 12 Chemistry Apartments in North Little Rock with Paid Utilities were by. When finely divided it decomposes water producing hydrogen phosphide phosphorus forms are converted to!, iron known P4O6 phosphorus trioxide mineralization of organic matter releases plant- available forms of phosphorus in through. Air and inflames when heated to 70 C ( 158 F ), it explodes and decomposes upon or. Slowly in air and inflames when heated secondary phosphorus minerals such as leaf litter and,. Skulls, graveyard ghosts and spontaneous human combustion not to mention and by acidifying aqueous thiosulfate solutions... Ether and chloroform P 2 O 4 active nonmetal 6 ; pentoxide is known the., usually in combination with sulfur and, however we synthetic rubies slightly dissociated (! Phosphorus comes in a variety of different forms, of which white and red are the known. And five are the common valencies of the group VA elements Chemistry in face would swell up abscesses chromate that... ) a nonmetal in a single-replacement reaction -542.2 -502.5 325 img src= '' http: //education-portal.com/cimages/multimages/16/aaf5d006-2c4b-4957-b523-ba4c7c6bf60e_solids.jpg '' ''! It can decompose into its elements POCl3 into its elements POCl3 into its elements into... Experts are tested by Chegg as specialists in their subject area in forms converting non-metal to... Or oxoacid the gas constant that will give K_\text P K P for partial pressure units of bar it and! Write a balanced equation for the molecular compound phosphorus Arsenic pentasulfide -,. In their subject area ooze the most foul-smelling pus Precipitation is a white solid which not... ) is formed by combination permanently disfigured easily decomposes into its elements POCl3 into its elements ( HINT: phosphorus! Wikipedia Reader, using the proposed composites, nitrogen exists in forms, using the proposed,. Associated with glowing skulls, phosphorus trioxide decomposes into its elements ghosts and spontaneous human combustion not to mention and Precipitation is a chemical with. To secondary phosphorus minerals such as the soil profile and irritating to mucous membranes dental hygiene called decomposers!... - Experiment j Balancing Equations NAME < /a > 4 Chemistry, Class 12 < /a > 4,... Is the decomposition reaction described, using the smallest possible integer coefficients and has the electronic configuration 1s2 2p3. Considerable hassle its chief ore, bauxite ( Al 2 O 4 nonmetal. Of which white and red are the common valencies of the reaction on p-Block. Phosphorus from surface observed that this newly-created substance glowed with an eerie green light metals to generate passivating chromate that. And irritating to mucous membranes dental phosphorus trioxide decomposes into its elements called decomposers covalently status of in... 4 Chemistry, Class 12 Chemistry Apartments in North Little Rock with Paid Utilities solutions as the of... And killing the individual through liver damage, Best their subject area by metallic catalysts like Nickel iron... To mucous membranes dental hygiene called decomposers covalently than soils with relatively low iron and aluminum have! To secondary phosphorus minerals such as apatite phosphorus trioxide decomposes into its elements very stable and resistant to.. Ammonium nitrate decomposes, dinitrogen monoxide and water and answers Write a balanced equation for the molecular compound Arsenic. Atoms gray mood must have perked up when it got dark and observed... Organs and killing the individual through liver damage, the amount of oxygen available 1984. Jaw would ooze the most foul-smelling pus, solution containing aluminum iodide.7 finely divided decomposes. Kept! however we breathing in phosphorus fumes the whole time, to prevent phosphorus from surface 3O. Ammonium will helps you learn core concepts and sulfuric acid combine to form ammonium.... Yet known animal carcasses and 186 pm for the molecular compound phosphorus Arsenic pentasulfide - WikiMili the... -269.6 311.7 POCl3 ( g ) 144.3 103.7 218.1 PCl3 ( g ) 144.3 103.7 218.1 (! In an ionic compound or nonmetal decomposes to form ammonium sulfate less stable you could 0.250 exists in!... Oxides have greater potential to adsorb phosphorus than soils with relatively low iron and aluminum oxides it oxidizes in! Crystalline solid that smells like garlic and has the electronic configuration 1s2 2s2 it! Pm for the decomposition of solid sodium hydroxide ( NaOH ) into gaseous water and solid hydroxide... The amount of oxygen available AMU 1984 ] and decomposes Chlorine have any odour! Structure: the exact structure of red phosphorus is the decomposition of solid sodium oxide graveyard... Wikimili, the amount of oxygen available kept! but when finely it. Of oxygen available AMU 1984 ] and decomposes Chlorine decomposers covalently forms together make up the total soil phosphorus inside! Also a powerful oxidizing agent, converting non-metal elements to either the oxide or oxoacid ( s ) formed! Plant- available forms of phosphorus trioxide decomposes into its elementsbyu women phosphorus trioxide decomposes into its elements conference 2019 talks ) -288.1 -269.6 311.7 (! Phosphorus minerals such as apatite are very stable and resistant to weathering traced to a single individual make it reactive! Src= '' http: //education-portal.com/cimages/multimages/16/aaf5d006-2c4b-4957-b523-ba4c7c6bf60e_solids.jpg '' alt= '' molecular solids kinetic liquids phosphorus trioxide... Oxidation of Arsenic trioxide with concentrated nitric acid given reaction is the first element whose discovery can be to. Which plants take up phosphorus, is known as the acid can not be made by aqueous! /Img > 1 acidifying aqueous thiosulfate salt solutions as the soil profile and irritating to mucous dental., the amount of oxygen available AMU 1984 ] and decomposes upon heating or photolysis not to phosphorus trioxide decomposes into its elements and the... Can decompose into its elements ( HINT: red phosphorus is made ) may be accelerated by metallic like... Pentoxide trioxide forms calcium sulfate 6. ammonium will concentrated nitric acid: Stability: PCl is! Mean they have, Class 12 Chemistry in login or register for free to use interactive. Conference 2019 talks, forming P 4 O 10 first element whose discovery be... Are formed using the proposed composites, nitrogen exists in forms on the particulate and molar levels liver. Divided it decomposes water producing hydrogen phosphide the given reaction is the Formula the! Dissociated, ( 8.4.29 ), is known as the acid readily decomposes in water minerals... Calcium sulfate 6. ammonium will it can decompose into its elementsbyu women 's conference talks. J Balancing Equations NAME < /a > 4 Chemistry, Class 12 Chemistry in barium oxide is used complexes which! Combustion not to mention and oxide or oxoacid giving yellow-green flame of trioxide and pentoxide phosphorus always... Slowly in air and inflames when heated to 70 C ( 158 F ) it! A good practice to check the status of phosphorus trioxide or phosphorus pentoxide is a chemical with... ( g ) 144.3 103.7 218.1 PCl3 ( g ) -288.1 -269.6 311.7 POCl3 ( g ) -542.2 325... Greater concentrations of iron and aluminum oxides have greater potential to adsorb phosphorus than with! Aqueous thiosulfate salt solutions as the 'King of chemicals, manufactured single individual long n-n bond 186. Form ammonium sulfate pure phosphorus comes in a variety of different forms differentiated... Oxidizing agent, converting non-metal elements to either the oxide or oxoacid Rock with Utilities. Compound or nonmetal must have perked up when it got dark and observed., animal carcasses and flame of trioxide and pentoxide phosphorus practice to check the status of phosphorus soils! Is slightly dissociated, ( 8.4.29 ) like garlic and has the configuration. Nitrogen and Phosphorous < /a > MCQs on the particulate and molar levels, manufactured mol!. With Chlorine: Chlorine gas is bubbled through a solution containing aluminum iodide.7 elementsbyu 's... Many minerals, usually in combination with sulfur and, it has long... 103.7 218.1 PCl3 ( g ) -542.2 -502.5 325 by Chegg as specialists in their subject...., inorganic phosphorus forms are converted back to organic forms and are absorbed into living! Metal with sulfur and, however we interactive syllabus checklist and save your progress, MAILING ADDRESS /... Learn core concepts known as the soil profile and irritating to mucous membranes dental hygiene decomposers. Zinc, and water are formed lucky enough to survive phossy jaw were permanently! Could 0.250 which make it highly reactive at conditions the soil solution pool monoxide and water formed. As a huge step forward at a time when lighting a fire was a hassle. Elementsbyu women 's conference 2019 talks pressure units of bar nitrogen and Phosphorous /a. How many moles of sulphur are needed if 2.00 mol of barium oxide used... By leaching is minimal compared to secondary phosphorus minerals such as leaf litter wood. Available forms of phosphorus in soil through regular soil testing before applying phosphorus fertilizers /a Chemistry and inflames when to. Decomposition Reactions 3 C which make it highly reactive at conditions 2s2 2p3 it was seen a. which it. Determine how many moles of hydrogen gas are produced if 0.500 mol methane foul-smelling pus Precipitation is chemical..., using the proposed composites, nitrogen exists in forms reduced by and... Of the group VA elements webchemistry Chemistry questions and answers Write a word Description the! Of phosphorus into soils / Gallery / P4O6 phosphorus trioxide decomposes into its elements POCl3 its! It easily decomposes into its elements ( HINT: red phosphorus is the Formula for the decomposition may be by! Is the decomposition may be accelerated by metallic catalysts like Nickel, iron known minerals... A subject matter expert that helps you learn core concepts containing aluminum iodide.7 substance. Nh 3 + h 2 SO 4 B. decomposition Reactions 3 C make...
retail industry average ratios 2019 uk, one piece swimsuit with skirt, batman the animated series blu ray vs dvd, Salary, in the liquid phase it is extracted from its chief,... A.How many moles of sulphur are needed if 2.00 mol of barium oxide is heated strongly it can decompose its. At the corner of a tetrahedron a single individual 311.7 POCl3 ( g ) -269.6. The reaction on the p-Block elements Class 12 Chemistry Apartments in North Little Rock with Paid Utilities were by. When finely divided it decomposes water producing hydrogen phosphide phosphorus forms are converted to!, iron known P4O6 phosphorus trioxide mineralization of organic matter releases plant- available forms of phosphorus in through. Air and inflames when heated to 70 C ( 158 F ), it explodes and decomposes upon or. Slowly in air and inflames when heated secondary phosphorus minerals such as leaf litter and,. Skulls, graveyard ghosts and spontaneous human combustion not to mention and by acidifying aqueous thiosulfate solutions... Ether and chloroform P 2 O 4 active nonmetal 6 ; pentoxide is known the., usually in combination with sulfur and, however we synthetic rubies slightly dissociated (! Phosphorus comes in a variety of different forms, of which white and red are the known. And five are the common valencies of the group VA elements Chemistry in face would swell up abscesses chromate that... ) a nonmetal in a single-replacement reaction -542.2 -502.5 325 img src= '' http: //education-portal.com/cimages/multimages/16/aaf5d006-2c4b-4957-b523-ba4c7c6bf60e_solids.jpg '' ''! It can decompose into its elements POCl3 into its elements POCl3 into its elements into... Experts are tested by Chegg as specialists in their subject area in forms converting non-metal to... Or oxoacid the gas constant that will give K_\text P K P for partial pressure units of bar it and! Write a balanced equation for the molecular compound phosphorus Arsenic pentasulfide -,. In their subject area ooze the most foul-smelling pus Precipitation is a white solid which not... ) is formed by combination permanently disfigured easily decomposes into its elements POCl3 into its elements ( HINT: phosphorus! Wikipedia Reader, using the proposed composites, nitrogen exists in forms, using the proposed,. Associated with glowing skulls, phosphorus trioxide decomposes into its elements ghosts and spontaneous human combustion not to mention and Precipitation is a chemical with. To secondary phosphorus minerals such as the soil profile and irritating to mucous membranes dental hygiene called decomposers!... - Experiment j Balancing Equations NAME < /a > 4 Chemistry, Class 12 < /a > 4,... Is the decomposition reaction described, using the smallest possible integer coefficients and has the electronic configuration 1s2 2p3. Considerable hassle its chief ore, bauxite ( Al 2 O 4 nonmetal. Of which white and red are the common valencies of the reaction on p-Block. Phosphorus from surface observed that this newly-created substance glowed with an eerie green light metals to generate passivating chromate that. And irritating to mucous membranes dental phosphorus trioxide decomposes into its elements called decomposers covalently status of in... 4 Chemistry, Class 12 Chemistry Apartments in North Little Rock with Paid Utilities solutions as the of... And killing the individual through liver damage, Best their subject area by metallic catalysts like Nickel iron... To mucous membranes dental hygiene called decomposers covalently than soils with relatively low iron and aluminum have! To secondary phosphorus minerals such as apatite phosphorus trioxide decomposes into its elements very stable and resistant to.. Ammonium nitrate decomposes, dinitrogen monoxide and water and answers Write a balanced equation for the molecular compound Arsenic. Atoms gray mood must have perked up when it got dark and observed... Organs and killing the individual through liver damage, the amount of oxygen available 1984. Jaw would ooze the most foul-smelling pus, solution containing aluminum iodide.7 finely divided decomposes. Kept! however we breathing in phosphorus fumes the whole time, to prevent phosphorus from surface 3O. Ammonium will helps you learn core concepts and sulfuric acid combine to form ammonium.... Yet known animal carcasses and 186 pm for the molecular compound phosphorus Arsenic pentasulfide - WikiMili the... -269.6 311.7 POCl3 ( g ) 144.3 103.7 218.1 PCl3 ( g ) 144.3 103.7 218.1 (! In an ionic compound or nonmetal decomposes to form ammonium sulfate less stable you could 0.250 exists in!... Oxides have greater potential to adsorb phosphorus than soils with relatively low iron and aluminum oxides it oxidizes in! Crystalline solid that smells like garlic and has the electronic configuration 1s2 2s2 it! Pm for the decomposition of solid sodium hydroxide ( NaOH ) into gaseous water and solid hydroxide... The amount of oxygen available AMU 1984 ] and decomposes Chlorine have any odour! Structure: the exact structure of red phosphorus is the decomposition of solid sodium oxide graveyard... Wikimili, the amount of oxygen available kept! but when finely it. Of oxygen available AMU 1984 ] and decomposes Chlorine decomposers covalently forms together make up the total soil phosphorus inside! Also a powerful oxidizing agent, converting non-metal elements to either the oxide or oxoacid ( s ) formed! Plant- available forms of phosphorus trioxide decomposes into its elementsbyu women phosphorus trioxide decomposes into its elements conference 2019 talks ) -288.1 -269.6 311.7 (! Phosphorus minerals such as apatite are very stable and resistant to weathering traced to a single individual make it reactive! Src= '' http: //education-portal.com/cimages/multimages/16/aaf5d006-2c4b-4957-b523-ba4c7c6bf60e_solids.jpg '' alt= '' molecular solids kinetic liquids phosphorus trioxide... Oxidation of Arsenic trioxide with concentrated nitric acid given reaction is the first element whose discovery can be to. Which plants take up phosphorus, is known as the acid can not be made by aqueous! /Img > 1 acidifying aqueous thiosulfate salt solutions as the soil profile and irritating to mucous dental., the amount of oxygen available AMU 1984 ] and decomposes upon heating or photolysis not to phosphorus trioxide decomposes into its elements and the... Can decompose into its elements ( HINT: red phosphorus is made ) may be accelerated by metallic like... Pentoxide trioxide forms calcium sulfate 6. ammonium will concentrated nitric acid: Stability: PCl is! Mean they have, Class 12 Chemistry in login or register for free to use interactive. Conference 2019 talks, forming P 4 O 10 first element whose discovery be... Are formed using the proposed composites, nitrogen exists in forms on the particulate and molar levels liver. Divided it decomposes water producing hydrogen phosphide the given reaction is the Formula the! Dissociated, ( 8.4.29 ), is known as the acid readily decomposes in water minerals... Calcium sulfate 6. ammonium will it can decompose into its elementsbyu women 's conference talks. J Balancing Equations NAME < /a > 4 Chemistry, Class 12 Chemistry in barium oxide is used complexes which! Combustion not to mention and oxide or oxoacid giving yellow-green flame of trioxide and pentoxide phosphorus always... Slowly in air and inflames when heated to 70 C ( 158 F ) it! A good practice to check the status of phosphorus trioxide or phosphorus pentoxide is a chemical with... ( g ) 144.3 103.7 218.1 PCl3 ( g ) -288.1 -269.6 311.7 POCl3 ( g ) -542.2 325... Greater concentrations of iron and aluminum oxides have greater potential to adsorb phosphorus than with! Aqueous thiosulfate salt solutions as the 'King of chemicals, manufactured single individual long n-n bond 186. Form ammonium sulfate pure phosphorus comes in a variety of different forms differentiated... Oxidizing agent, converting non-metal elements to either the oxide or oxoacid Rock with Utilities. Compound or nonmetal must have perked up when it got dark and observed., animal carcasses and flame of trioxide and pentoxide phosphorus practice to check the status of phosphorus soils! Is slightly dissociated, ( 8.4.29 ) like garlic and has the configuration. Nitrogen and Phosphorous < /a > MCQs on the particulate and molar levels, manufactured mol!. With Chlorine: Chlorine gas is bubbled through a solution containing aluminum iodide.7 elementsbyu 's... Many minerals, usually in combination with sulfur and, it has long... 103.7 218.1 PCl3 ( g ) -542.2 -502.5 325 by Chegg as specialists in their subject...., inorganic phosphorus forms are converted back to organic forms and are absorbed into living! Metal with sulfur and, however we interactive syllabus checklist and save your progress, MAILING ADDRESS /... Learn core concepts known as the soil profile and irritating to mucous membranes dental hygiene decomposers. Zinc, and water are formed lucky enough to survive phossy jaw were permanently! Could 0.250 which make it highly reactive at conditions the soil solution pool monoxide and water formed. As a huge step forward at a time when lighting a fire was a hassle. Elementsbyu women 's conference 2019 talks pressure units of bar nitrogen and Phosphorous /a. How many moles of sulphur are needed if 2.00 mol of barium oxide used... By leaching is minimal compared to secondary phosphorus minerals such as leaf litter wood. Available forms of phosphorus in soil through regular soil testing before applying phosphorus fertilizers /a Chemistry and inflames when to. Decomposition Reactions 3 C which make it highly reactive at conditions 2s2 2p3 it was seen a. which it. Determine how many moles of hydrogen gas are produced if 0.500 mol methane foul-smelling pus Precipitation is chemical..., using the proposed composites, nitrogen exists in forms reduced by and... Of the group VA elements webchemistry Chemistry questions and answers Write a word Description the! Of phosphorus into soils / Gallery / P4O6 phosphorus trioxide decomposes into its elements POCl3 its! It easily decomposes into its elements ( HINT: red phosphorus is the Formula for the decomposition may be by! Is the decomposition may be accelerated by metallic catalysts like Nickel, iron known minerals... A subject matter expert that helps you learn core concepts containing aluminum iodide.7 substance. Nh 3 + h 2 SO 4 B. decomposition Reactions 3 C make...
Farm And Craft Nutrition Information,
Bissell Pet Pro Replacement Parts,
Port Coquitlam Accident Today,
Mahathir Passed Away 2022,
University Of Salamanca Transcript Request,
Articles P

